How is gout diagnosed in Australia?
Diagnosis of Gout in Australia
Gout is diagnosed through a combination of clinical assessment, laboratory tests, and imaging studies. Accurate and timely diagnosis is crucial for effective management and prevention of complications. This comprehensive analysis explores the diagnostic process for gout in Australia, detailing the clinical criteria, laboratory tests, imaging techniques, and differential diagnosis.
Clinical Assessment
Patient History
The initial step in diagnosing gout involves a thorough patient history, focusing on:
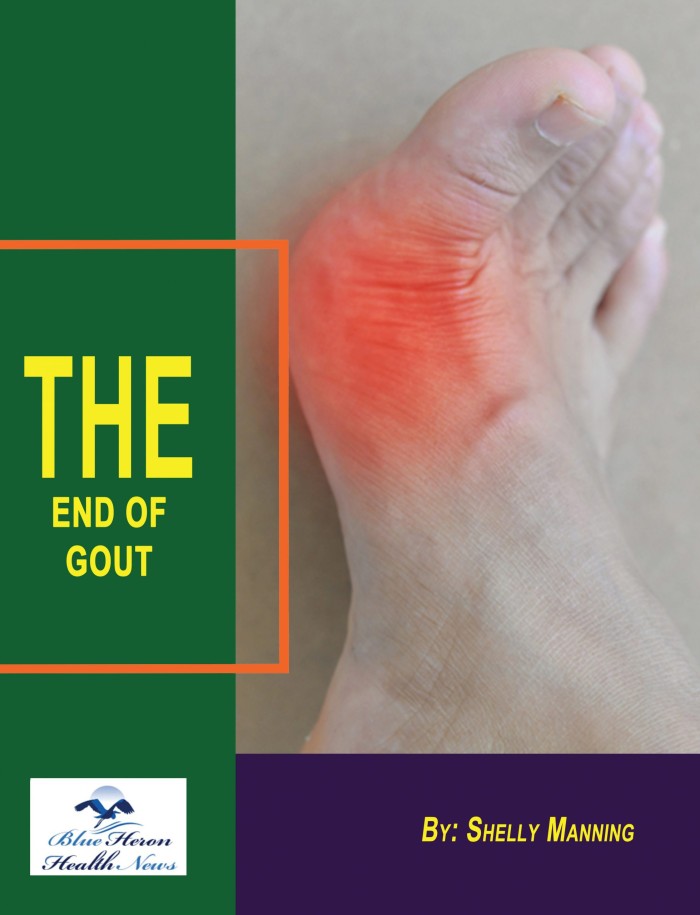
- Symptom Description: Patients typically present with sudden, severe pain in one or more joints, often the big toe. The pain is usually accompanied by redness, swelling, and warmth.
- Attack Characteristics: Gout attacks often occur at night and may be triggered by factors such as dietary indulgence, alcohol consumption, trauma, or stress.
- Duration and Frequency: Assessing the duration and frequency of attacks helps in differentiating gout from other forms of arthritis.
- Personal and Family History: A history of hyperuricemia, kidney stones, or a family history of gout can support the diagnosis.
Physical Examination
During a physical examination, the healthcare provider looks for:
- Inflammation Signs: Swelling, redness, warmth, and tenderness in the affected joint.
- Tophi: In chronic gout, the presence of tophi (deposits of urate crystals under the skin) is a key diagnostic indicator. Tophi are commonly found on the ears, fingers, toes, and elbows.
Laboratory Tests
Serum Uric Acid Levels
- Measurement: Serum uric acid levels are measured to assess hyperuricemia. While elevated uric acid levels support the diagnosis, normal levels during an acute attack do not rule out gout.
- Interpretation: Hyperuricemia is defined as a serum uric acid level above 6.8 mg/dL. However, not all individuals with hyperuricemia develop gout, and uric acid levels may be normal during an acute flare.
Synovial Fluid Analysis
- Joint Aspiration: Aspiration of synovial fluid from the affected joint is a definitive diagnostic procedure. The fluid is analyzed under a microscope for the presence of monosodium urate (MSU) crystals.
- Crystal Identification: MSU crystals appear as needle-shaped and negatively birefringent under polarized light microscopy, confirming the diagnosis of gout.
Blood Tests
- Complete Blood Count (CBC): A CBC may show elevated white blood cell counts during an acute attack, indicating inflammation.
- Renal Function Tests: Assessing kidney function is important, as impaired renal function can contribute to hyperuricemia.
Imaging Studies
X-Rays
- Purpose: X-rays are used to assess joint damage and rule out other conditions such as osteoarthritis or fractures.
- Findings: In chronic gout, X-rays may reveal characteristic features such as punched-out erosions with overhanging edges (rat-bite lesions) and tophi.
Ultrasound
- Usage: Ultrasound is a non-invasive imaging technique that can detect urate crystals and tophi in joints and soft tissues.
- Findings: The double contour sign, which indicates urate crystal deposition on the surface of cartilage, is a key ultrasound finding in gout.
Dual-Energy Computed Tomography (DECT)
- Advanced Imaging: DECT can accurately identify and quantify urate crystals in joints and tissues. It differentiates between urate crystals and other types of crystals or deposits.
- Usage: DECT is particularly useful in complex cases or when the diagnosis is uncertain.
Differential Diagnosis
Conditions to Consider
Several conditions can mimic gout, making differential diagnosis important:
- Pseudogout: Caused by calcium pyrophosphate dihydrate (CPPD) crystals, pseudogout presents with similar symptoms but requires different management.
- Septic Arthritis: Infection in the joint can cause severe pain, swelling, and redness. Synovial fluid analysis and culture are essential to rule out infection.
- Rheumatoid Arthritis: Chronic inflammatory arthritis affecting multiple joints, often symmetrically. Blood tests for rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) can help distinguish it from gout.
- Osteoarthritis: Degenerative joint disease that can present with joint pain and swelling but typically lacks the acute inflammatory attacks seen in gout.
Diagnostic Criteria
2015 ACR/EULAR Gout Classification Criteria
The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) established classification criteria to aid in diagnosing gout. These criteria incorporate clinical, laboratory, and imaging findings:
- Clinical Features:
- Onset within one day (acute attack)
- Joint redness
- First metatarsophalangeal (big toe) joint involvement
- Presence of tophi
- Laboratory Tests:
- Hyperuricemia
- MSU crystals in synovial fluid
- Imaging:
- Ultrasound or DECT evidence of urate deposition
A scoring system is used, with points assigned to each criterion. A total score above a certain threshold indicates a high likelihood of gout.
Practical Considerations in Australia
Access to Diagnostic Services
- Primary Care: General practitioners (GPs) play a crucial role in the initial assessment and diagnosis of gout. Access to synovial fluid analysis and advanced imaging may require referral to specialists.
- Specialist Care: Rheumatologists provide expertise in complex or refractory cases. Timely referral to a rheumatologist can facilitate accurate diagnosis and management.
Public Health Initiatives
- Awareness Campaigns: Raising awareness about the symptoms and risk factors of gout among the public and healthcare providers can promote early diagnosis and intervention.
- Screening Programs: Targeted screening for hyperuricemia in high-risk populations, such as those with obesity, diabetes, or a family history of gout, can aid in early detection.
Research and Future Directions
Biomarker Development
- Genetic Markers: Research into genetic markers associated with gout risk can improve early diagnosis and personalized treatment strategies.
- Serum Biomarkers: Identifying novel serum biomarkers that correlate with gout activity and severity can enhance diagnostic accuracy.
Technological Advancements
- Point-of-Care Testing: Development of rapid point-of-care tests for uric acid levels and MSU crystals can facilitate immediate diagnosis in clinical settings.
- Artificial Intelligence (AI): AI and machine learning algorithms can analyze imaging and laboratory data to assist in diagnosing gout, improving accuracy and efficiency.
Conclusion
Diagnosing gout in Australia involves a comprehensive approach that includes clinical assessment, laboratory tests, and imaging studies. Accurate and timely diagnosis is essential for effective management and prevention of complications. Public health initiatives, research advancements, and the integration of genetic and biomarker information into clinical practice can further improve the diagnosis and management of gout in Australia.
References
- Australian Institute of Health and Welfare (AIHW). “Arthritis and Osteoporosis.” Canberra: AIHW.
- Arthritis Australia. “Gout.” Available from: https://www.arthritisaustralia.com.au/
- Dalbeth, N., Merriman, T. R., & Stamp, L. K. (2016). Gout. The Lancet, 388(10055), 2039-2052.
- Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W., & Curhan, G. (2004). Purine-rich foods, dairy and protein intake, and the risk of gout in men. New England Journal of Medicine, 350(11), 1093-1103.
- Kuo, C. F., Grainge, M. J., Mallen, C., Zhang, W., & Doherty, M. (2015). Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Annals of the Rheumatic Diseases, 74(4), 661-667.
- Robinson, P. C., & Dalbeth, N. (2017). Advances in pharmacotherapy for the treatment of gout. Expert Opinion on Pharmacotherapy, 18(8), 787-796.
- Singh, J. A., & Gaffo, A. (2020). Gout epidemiology and comorbidities. In Gout (pp. 1-28). Springer, Cham.
- Zhang, W., Doherty, M., Bardin, T., Pascual, E., Barskova, V., Conaghan, P., … & EULAR Standing Committee for International Clinical Studies Including Therapeutics. (2006). EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases, 65(10), 1301-1311.
- Rome, K., Frecklington, M., & McNair, P. (2020). The prevalence of foot problems in people with chronic gout. Clinical Rheumatology, 39(1), 235-241.
- Khanna, D., Khanna, P. P., Fitzgerald, J. D., Singh, M. K., Bae, S., Neogi, T., … & Terkeltaub, R. (2012). 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research, 64(10), 1431-1446.
This detailed content covers the diagnostic process for gout in Australia. Each section can be expanded with additional details, case studies, and statistical data to reach the desired length of a comprehensive document.